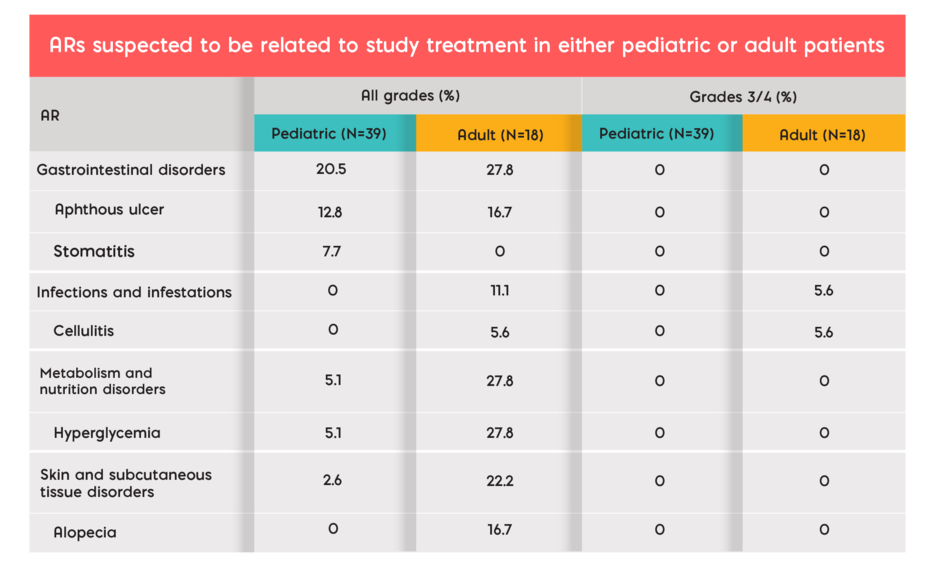
Serious and Common Adverse Reactions
Safety profile in PIK3CA-Related Overgrowth Spectrum (PROS)1
EPIK-P1 | ||
AR (≥5% of patients) | All grades (%) | Grades 3/4 (%) |
Diarrhea | 16 | 0 |
Stomatitisa | 16 | 0 |
Hyperglycemia | 12 | 0 |
Eczema | 7 | 0 |
Dry skin | 7 | 0 |
Cellulitis | 5 | 3.5 |
Alopecia | 5 | 0 |
Headache | 5 | 0 |
Laboratory abnormality (>20% of patients)b | All grades (%) | Grades 3/4 (%) |
Chemistry | ||
Decreased calcium (corrected) | 60 | 0 |
Decreased phosphate | 59 | 5e |
Increased glucosec | 56 | 11e |
Increased glycosylated hemoglobin (HbA1c)d | 38d | NAd |
Increased creatinine | 31 | 0 |
Increased bilirubin | 29 | 2e |
Increased potassium | 24 | 0 |
Hematology | ||
Decreased leukocyte | 22 | 0 |
Decreased hemoglobin | 20 | 6e |
Decreased lymphocyte | 20 | 0 |
Grading according to CTCAE version 4.03.
aStomatitis, including stomatitis and aphthous ulcer.
bThe denominator used to calculate the rate varied from 9 to 50 based on the number of patients with a baseline value and at least 1 posttreatment value.
cGlucose increase is an expected laboratory abnormality of PI3K inhibition.
dNo CTCAE grade available. For HbA1c, baseline values increasing posttreatment to a value above the upper limit of the normal range (≥5.7%) are considered increased.
eNo grade 4 laboratory abnormalities were reported.
AR, adverse reaction; NA, not available; CTCAE, Common Terminology Criteria for Adverse Events; PI3K, phosphatidylinositol-3-kinase.
EPIK-P1 + EPIK-P3
Longer-term follow-up did not show any new safety signals2
Since initiating VIJOICE, patients (N=57) were exposed to treatment for a median duration of 42.0 months (range: 3.4-74.8 months)
22.8% (n=13) of patients were exposed for >60 months
Most pediatric patients experienced normal growth and development2
EPIK-P1
The most commonly reported ARs were diarrhea (16%), stomatitis (16%), and hyperglycemia (12%)
All ARs occurring in ≥10% of patients were mild to moderate (grade 1 or 2)
Serious ARs occurred in 12% of patients who received VIJOICE. Dehydration (n=2) and cellulitis (n=2) were the only serious ARs that occurred in multiple patients
Additional safety data
5% of patients required dose reductions due to ARs. Alopecia, memory impairment, and soft tissue infection were the only ARs that required dose reduction
Dose interruption due to an AR occurred in 11% of patients. Vomiting (n=2) and dizziness (n=2) were the only ARs that required dose interruption in 2 or more patients
ARs caused by targeted therapies are generally reversible3,4
Patient Management Guide

Managing Your Patient on VIJOICE
Download this guide to help you manage potential adverse reactions during treatment.